Abstract
Introduction: Autologous stem cell transplantation has been a staple treatment modality in patients with multiple myeloma for more than 30 years. Multiple studies have shown increased survival among patients who undergo transplant when compared to those who receive chemotherapy alone, even amongst elderly patients. Despite the efficacy associated with transplant among populations as a whole, individual response to therapy is variable and difficult to predict. Recent studies however have demonstrated that achieving minimal residual disease (MRD) negativity is associated with increased survival in patients with multiple myeloma. In this study, we performed a retrospective analysis on patients with multiple myeloma who underwent autologous stem cell transplantation and investigated potential markers to predict post-transplant MRD status.
Patients and Methods: Patients with a diagnosis of multiple myeloma that underwent treatment with high-dose melphalan followed by autologous stem cell transplantation at the Indiana University Simon Cancer Center between 2019-2020 were included in the analysis. Patient demographics, disease characteristics, pre-transplant and post-transplant laboratory values, and approximately day +100 post-transplant bone marrow sample results were collected. MRD analysis on post-transplant bone marrow aspirations was performed using 8 color flow cytometry panel with a total of 10 markers. The limits of quantification and detection were calculated at 5X10 -6 and 2X10 -6, respectively. Post-transplant data was analyzed to determine MRD status. MRD negativity was defined as having no identifiable M protein via serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) and having negative MRD on post-transplant bone marrow biopsy testing. Patients with insufficient data to determine post-transplant MRD status were excluded from the analysis. Univariate logistic regression was performed to assess the association of pre-transplant variables with post-transplant MRD status. Multivariate logistic regression model was utilized to analyze markers with a p-value <0.25 in univariate analysis.
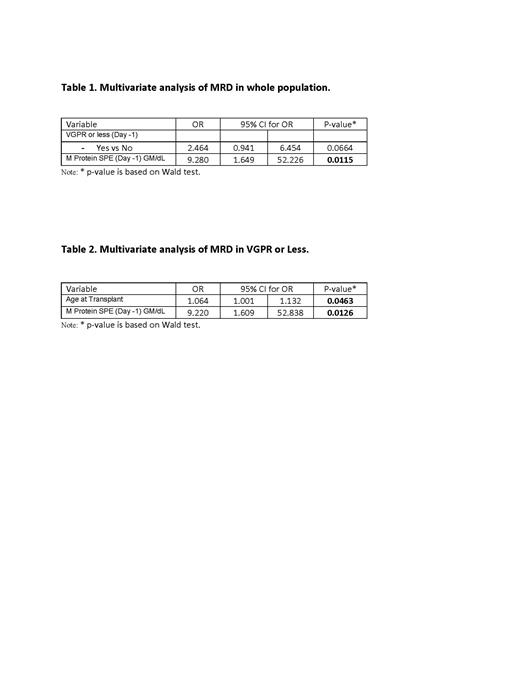
Results: 133 Patients were included in the analysis with average age at transplant being 60.84 years (range 32.18 years-78.13 years). 83/133 (62.41%) patients were male and 118/133 (88.72%) patients were white. 84/133 (63.16%) patients had achieved a VGPR or less according to the International Myeloma Working Group (IMWG) response criteria prior to transplant. Among all patients, age at transplant, gender, race, body mass index, glomerular filtration rate on day -1, serum albumin on day -1, kappa/lambda ratio on day -1, melphalan dose received, and multiple myeloma immunoglobulin subtype were not associated with response to therapy. Pre-transplant M protein positivity was associated with a higher likelihood of post-transplant MRD positive status with an odds ratio of 24.318 (p<0.0001). VGPR status or less on day -1 was associated with an increased post-transplant MRD positive status with an odds ratio of 6.223 (p<0.0001) however was not found to be statistically significant following multivariate analysis (p=0.0664). When restricting analysis to include only patients at VGPR status or less prior to transplant, pre-transplant M protein positivity and increased age at transplant were associated with increased likelihood of MRD positive status with odd ratios of 9.000 (p=0.0121) and 1.066 (p=0.0366) respectively. Both variables were shown to be statistically significant following multivariate analysis.
Conclusions: Detectable levels of pre-transplant M protein via serum protein electrophoresis is associated with an increased likelihood of having positive minimal residual disease following autologous stem cell transplantation in multiple myeloma. Age at transplant does not predict minimal residual disease status among all patients undergoing transplant, however increased age at transplant may be associated with inferior outcomes in patients achieving a VGPR or less prior to transplantation.
Suvannasankha: The Veteran's Affair: Patents & Royalties; Karyopharm: Consultancy, Research Funding; Regeneron: Research Funding; Sutro: Research Funding; Glaxo Smith Kline: Consultancy, Research Funding; Janssen Oncology: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Abonour: Celgene-BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jensen: Honoraria, Research Funding; Takeda: Research Funding; GSK: Consultancy, Honoraria, Research Funding. Abu Zaid: Syndax: Consultancy, Research Funding; Pieris: Current equity holder in publicly-traded company; Incyte: Research Funding; Pharamcyclic: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal